
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| Thermodynamics |
|---|
 |
An isentropic process is an idealized thermodynamic process that is both adiabatic and reversible.[1][2][3][4][5][6][excessive citations] The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes.[7] This process is idealized because reversible processes do not occur in reality; thinking of a process as both adiabatic and reversible would show that the initial and final entropies are the same, thus, the reason it is called isentropic (entropy does not change). Thermodynamic processes are named based on the effect they would have on the system (ex. isovolumetric: constant volume, isenthalpic: constant enthalpy). Even though in reality it is not necessarily possible to carry out an isentropic process, some may be approximated as such.
The word "isentropic" derives from the process being one in which the entropy of the system remains unchanged. In addition to a process which is both adiabatic and reversible, this can also occur in a system where the work done on the system includes friction internal to the system, and heat is withdrawn from the system sufficient to compensate for it so as to leave the entropy unchanged.[8] However, in relation to the Universe, its entropy would increase as a result, in agreement with the Second Law of Thermodynamics.[citation needed]
Background
The second law of thermodynamics states[9][10] that
where is the amount of energy the system gains by heating, is the temperature of the surroundings, and is the change in entropy. The equal sign refers to a reversible process, which is an imagined idealized theoretical limit, never actually occurring in physical reality, with essentially equal temperatures of system and surroundings.[11][12] For an isentropic process, if also reversible, there is no transfer of energy as heat because the process is adiabatic; δQ = 0. In contrast, if the process is irreversible, entropy is produced within the system; consequently, in order to maintain constant entropy within the system, energy must be simultaneously removed from the system as heat.
For reversible processes, an isentropic transformation is carried out by thermally "insulating" the system from its surroundings. Temperature is the thermodynamic conjugate variable to entropy, thus the conjugate process would be an isothermal process, in which the system is thermally "connected" to a constant-temperature heat bath.
Isentropic processes in thermodynamic systems
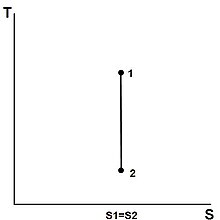
The entropy of a given mass does not change during a process that is internally reversible and adiabatic. A process during which the entropy remains constant is called an isentropic process, written or .[13] Some examples of theoretically isentropic thermodynamic devices are pumps, gas compressors, turbines, nozzles, and diffusers.
Isentropic efficiencies of steady-flow devices in thermodynamic systems
Most steady-flow devices operate under adiabatic conditions, and the ideal process for these devices is the isentropic process. The parameter that describes how efficiently a device approximates a corresponding isentropic device is called isentropic or adiabatic efficiency.[13]
Isentropic efficiency of turbines:
Isentropic efficiency of compressors:
Isentropic efficiency of nozzles:
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk























