A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
An extinction event (also known as a mass extinction or biotic crisis) is a widespread and rapid decrease in the biodiversity on Earth. Such an event is identified by a sharp fall in the diversity and abundance of multicellular organisms. It occurs when the rate of extinction increases with respect to the background extinction rate[1] and the rate of speciation. Estimates of the number of major mass extinctions in the last 540 million years range from as few as five to more than twenty. These differences stem from disagreement as to what constitutes a "major" extinction event, and the data chosen to measure past diversity.
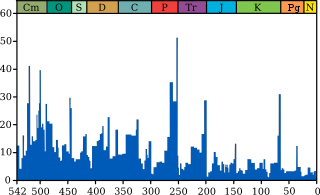
The "Big Five" mass extinctions
In a landmark paper published in 1982, Jack Sepkoski and David M. Raup identified five particular geological intervals with excessive diversity loss.[2] They were originally identified as outliers on a general trend of decreasing extinction rates during the Phanerozoic,[3] but as more stringent statistical tests have been applied to the accumulating data, it has been established that in the current, Phanerozoic Eon, multicellular animal life has experienced at least five major and many minor mass extinctions.[4] The "Big Five" cannot be so clearly defined, but rather appear to represent the largest (or some of the largest) of a relatively smooth continuum of extinction events.[3] All of the five in the Phanerozoic Eon were anciently preceded by the presumed far more extensive mass extinction of microbial life during the Great Oxidation Event (aka Oxygen Catastrophe) early in the Proterozoic Eon. At the end of the Ediacaran and just before the Cambrian explosion, yet another Proterozoic extinction event (of unknown magnitude) is speculated to have ushered in the Phanerozoic.[5]
| 1 | Ordovician–Silurian extinction events | 445–444 Ma |
| End Ordovician or O–S, just prior to and at the Ordovician–Silurian transition. Two events occurred that killed off 27% of all families, 57% of all genera and 85% of all species.[6] Together they are ranked by many scientists as the second-largest of the five major extinctions in Earth's history in terms of percentage of genera that became extinct.
In May 2020, studies suggested that the causes of the mass extinction were global warming, related to volcanism, and anoxia, and not, as considered earlier, cooling and glaciation.[7][8] However, this is at odds with numerous previous studies, which have indicated global cooling as the primary driver.[9] Most recently, the deposition of volcanic ash has been suggested to be the trigger for reductions in atmospheric carbon dioxide leading to the glaciation and anoxia observed in the geological record.[10]
| ||
| 2 | Late Devonian extinctions | 372–359 Ma |
| The Late Devonian extinctions were a series of events that occupied much of the Late Devonian up to the Devonian–Carboniferous transition. The Late Devonian was an interval of high diversity loss, concentrated into two extinction events.
The largest extinction was the Kellwasser Event (Frasnian-Famennian, or F-F, 372 Ma), an extinction event at the end of the Frasnian, about midway through the Late Devonian. This extinction annihilated coral reefs and numerous tropical benthic (seabed-living) animals such as jawless fish, brachiopods, and trilobites. The other major extinction was the Hangenberg Event (Devonian-Carboniferous, or D-C, 359 Ma), which brought an end to the Devonian as a whole. This extinction wiped out the armored placoderm fish and nearly led to the extinction of the newly evolved ammonoids. These two closely spaced extinction events collectively eliminated about 19% of all families, 50% of all genera[6] and at least 70% of all species.[11] Sepkoski and Raup (1982)[2] did not initially consider the Late Devonian extinction interval (Givetian, Frasnian, and Famennian stages) to be statistically significant.[2] Regardless, later studies have affirmed the strong ecological impacts of the Kellwasser and Hangenberg Events.[12]
| ||
| 3 | Permian–Triassic extinction event | 252 Ma |
 The End Permian extinction or the "Great Dying" occurred at the Permian–Triassic transition.[13] It was the Phanerozoic Eon's largest extinction: 53% of marine families died, 84% of marine genera, about 81% of all marine species[14] and an estimated 70% of terrestrial vertebrate species.[15] This is also the largest known extinction event for insects.[16] The highly successful marine arthropod, the trilobite, became extinct. The evidence regarding plants is less clear, but new taxa became dominant after the extinction.[17] The "Great Dying" had enormous evolutionary significance: On land, it ended the primacy of early synapsids. The recovery of vertebrates took 30 million years,[18] but the vacant niches created the opportunity for archosaurs to become ascendant. In the seas, the percentage of animals that were sessile (unable to move about) dropped from 67% to 50%. The whole late Permian was a difficult time, at least for marine life, even before the P–T boundary extinction. More recent research has indicated that the End-Capitanian extinction event that preceded the "Great Dying" likely constitutes a separate event from the P–T extinction; if so, it would be larger than some of the "Big Five" extinction events.
| ||
| 4 | Triassic–Jurassic extinction event | 201.3 Ma |
| The End Triassic extinction marks the Triassic–Jurassic transition. About 23% of all families, 48% of all genera (20% of marine families and 55% of marine genera) and 70% to 75% of all species became extinct.[6] Most non-dinosaurian archosaurs, most therapsids, and most of the large amphibians were eliminated, leaving dinosaurs with little terrestrial competition. Non-dinosaurian archosaurs continued to dominate aquatic environments, while non-archosaurian diapsids continued to dominate marine environments. The Temnospondyl lineage of large amphibians also survived until the Cretaceous in Australia (e.g., Koolasuchus).
| ||
| 5 | Cretaceous–Paleogene extinction event | 66 Ma |
 The End Cretaceous extinction, or the K–Pg extinction (formerly K–T extinction) occurred at the Cretaceous (Maastrichtian) – Paleogene (Danian) transition.[19] The event was formerly called the Cretaceous-Tertiary or K–T extinction or K–T boundary; it is now officially named the Cretaceous–Paleogene (or K–Pg) extinction event. About 17% of all families, 50% of all genera[6] and 75% of all species became extinct.[2] In the seas all the ammonites, plesiosaurs and mosasaurs disappeared and the percentage of sessile animals was reduced to about 33%. All non-avian dinosaurs became extinct during that time.[20] The boundary event was severe with a significant amount of variability in the rate of extinction between and among different clades. Mammals, descended from the synapsids, and birds, a side-branch of the theropod dinosaurs, emerged as the two predominant clades of terrestrial tetrapods. | ||
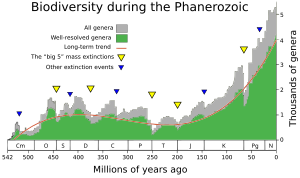
Despite the common presentation focusing only on these five events, no measure of extinction shows any definite line separating them from the many other Phanerozoic extinction events that appear only slightly lesser catastrophies; further, using different methods of calculating an extinction's impact can lead to other events featuring in the top five.[21]
Fossil records of older events are more difficult to interpret. This is because:
- Older fossils are harder to find as they are usually buried at a considerable depth.
- Dating of older fossils is more difficult.
- Productive fossil beds are researched more than unproductive ones, therefore leaving certain periods unresearched.
- Prehistoric environmental events can disturb the deposition process.
- Marine fossils tend to be better preserved than their more sought-after land-based counterparts, but the deposition and preservation of fossils on land is more erratic.[22]
It has been suggested that the apparent variations in marine biodiversity may actually be an artifact, with abundance estimates directly related to quantity of rock available for sampling from different time periods.[23] However, statistical analysis shows that this can only account for 50% of the observed pattern,[citation needed] and other evidence such as fungal spikes (geologically rapid increase in fungal abundance) provides reassurance that most widely accepted extinction events are real. A quantification of the rock exposure of Western Europe indicates that many of the minor events for which a biological explanation has been sought are most readily explained by sampling bias.[24]
Sixth mass extinction
Research completed after the seminal 1982 paper (Sepkoski and Raup) has concluded that a sixth mass extinction event is ongoing due to human activities:
| 6 | Holocene extinction | currently ongoing |
| Extinctions have occurred at over 1,000 times the background extinction rate since 1900, and the rate is increasing.[25][26][a] The mass extinction is a result of human activity (an ecocide)[28][29][30][31] driven by population growth and overconsumption of the earth's natural resources.[b] The 2019 global biodiversity assessment by IPBES asserts that out of an estimated 8 million species, 1 million plant and animal species are currently threatened with extinction.[33][34][35][36] In late 2021, WWF Germany suggested that over a million species could go extinct within a decade in the "largest mass extinction event since the end of the dinosaur age."[37] A 2023 study published in PNAS concluded that at least 73 genera of animals have gone extinct since 1500. If humans had never existed, it would have taken 18,000 years for the same genera to have disappeared naturally, the report states.[38][39][40] | ||
Extinctions by severity
Extinction events can be tracked by several methods, including geological change, ecological impact, extinction vs. origination (speciation) rates, and most commonly diversity loss among taxonomic units. Most early papers used families as the unit of taxonomy, based on compendiums of marine animal families by Sepkoski (1982, 1992).[41][42] Later papers by Sepkoski and other authors switched to genera, which are more precise than families and less prone to taxonomic bias or incomplete sampling relative to species.[43] These are several major papers estimating loss or ecological impact from fifteen commonly-discussed extinction events. Different methods used by these papers are described in the following section. The "Big Five" mass extinctions are bolded.
| Extinction name | Age (Ma) |
Sepkoski (1996)[44] Multiple-interval genera |
Bambach (2006)[45] |
McGhee et al. (2013)[12] | Stanley (2016)[14] | |
|---|---|---|---|---|---|---|
| Taxonomic loss |
Ecological ranking | |||||
| Late Ordovician (Ashgillian / Hirnantian) | 445–444 | ~49% | 57% (40%, 31%) |
52% | 7 | 42–46% |
| Lau event (Ludfordian) | 424 | ~23% | – | 9% | 9 | – |
| Kačák Event (Eifelian) | 388~ | ~24% | – | 32% | 9 | – |
| Taghanic Event (Givetian) | 384~ | ~30% | 28.5% | 36% | 8 | – |
| Late Devonian/Kellwasser event (Frasnian) | 372 | ~35% | 34.7% | 40% | 4 | 16–20% |
| End-Devonian/Hangenberg event (Famennian) | 359 | ~28% | 31% | 50% | 7 | <13% |
| Serpukhovian | 330–325~ | ~23% | 31% | 39% | 6 | 13–15% |
| Capitanian | 260 | ~47% | 48% | 25% | 5 | 33–35% |
| Permian–Triassic (Changhsingian) | 252 | ~58% | 55.7% | 83% | 1 | 62% |
| Triassic–Jurassic (Rhaetian) | 201 | ~37% | 47% | 73% | 3 | N/A |
| Pliensbachian-Toarcian | 186–178 | ~14% | 25%, 20% | – | – | – |
| End-Jurassic (Tithonian) | 145 | ~18% | 20% | – | – | – |
| Cenomanian-Turonian | 94 | ~15% | 25% | – | – | – |
| Cretaceous–Paleogene (Maastrichtian) | 66 | ~39% | 40–47% | 40% | 2 | 38–40% |
| Eocene–Oligocene | 34 | ~11% | 15.6% | – | – | – |
a Graphed but not discussed by Sepkoski (1996), considered continuous with the Late Devonian mass extinction
b At the time considered continuous with the end-Permian mass extinction
c Includes late Norian time slices
d Diversity loss of both pulses calculated together
e Pulses extend over adjacent time slices, calculated separately
f Considered ecologically significant, but not analyzed directly
g Excluded due to a lack of consensus on Late Triassic chronology
The study of major extinction events
Breakthrough studies in the 1980s–1990s

For much of the 20th century, the study of mass extinctions was hampered by insufficient data. Mass extinctions, though acknowledged, were considered mysterious exceptions to the prevailing gradualistic view of prehistory, where slow evolutionary trends define faunal changes. The first breakthrough was published in 1980 by a team led by Luis Alvarez, who discovered trace metal evidence for an asteroid impact at the end of the Cretaceous period. The Alvarez hypothesis for the end-Cretaceous extinction gave mass extinctions, and catastrophic explanations, newfound popular and scientific attention.[46]
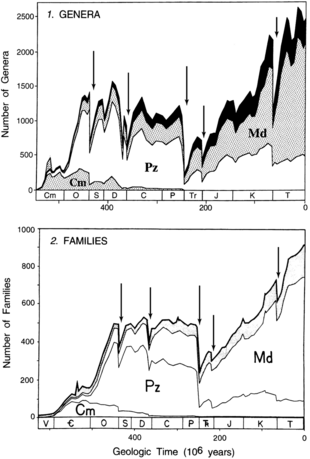
Another landmark study came in 1982, when a paper written by David M. Raup and Jack Sepkoski was published in the journal Science.[2] This paper, originating from a compendium of extinct marine animal families developed by Sepkoski,[41] identified five peaks of marine family extinctions which stand out among a backdrop of decreasing extinction rates through time. Four of these peaks were statistically significant: the Ashgillian (end-Ordovician), Late Permian, Norian (end-Triassic), and Maastrichtian (end-Cretaceous). The remaining peak was a broad interval of high extinction smeared over the later half of the Devonian, with its apex in the Frasnian stage.[2]
Through the 1980s, Raup and Sepkoski continued to elaborate and build upon their extinction and origination data, defining a high-resolution biodiversity curve (the "Sepkoski curve") and successive evolutionary faunas with their own patterns of diversification and extinction.[47][48][49][50][51][52] Though these interpretations formed a strong basis for subsequent studies of mass extinctions, Raup and Sepkoski also proposed a more controversial idea in 1984: a 26-million-year periodic pattern to mass extinctions.[53] Two teams of astronomers linked this to a hypothetical brown dwarf in the distant reaches of the solar system, inventing the "Nemesis hypothesis" which has been strongly disputed by other astronomers.
Around the same time, Sepkoski began to devise a compendium of marine animal genera, which would allow researchers to explore extinction at a finer taxonomic resolution. He began to publish preliminary results of this in-progress study as early as 1986, in a paper which identified 29 extinction intervals of note.[51] By 1992, he also updated his 1982 family compendium, finding minimal changes to the diversity curve despite a decade of new data.[42][54] In 1996, Sepkoski published another paper which tracked marine genera extinction (in terms of net diversity loss) by stage, similar to his previous work on family extinctions. The paper filtered its sample in three ways: all genera (the entire unfiltered sample size), multiple-interval genera (only those found in more than one stage), and "well-preserved" genera (excluding those from groups with poor or understudied fossil records). Diversity trends in marine animal families were also revised based on his 1992 update.[44]
Revived interest in mass extinctions led many other authors to re-evaluate geological events in the context of their effects on life.[55] A 1995 paper by Michael Benton tracked extinction and origination rates among both marine and continental (freshwater & terrestrial) families, identifying 22 extinction intervals and no periodic pattern.[56] Overview books by O.H. Walliser (1996) and A. Hallam and P.B. Wignall (1997) summarized the new extinction research of the previous two decades.[57][58] One chapter in the former source lists over 60 geological events which could conceivably be considered global extinctions of varying sizes.[59] These texts, and other widely circulated publications in the 1990s, helped to establish the popular image of mass extinctions as a "big five" alongside many smaller extinctions through prehistory.
New data on genera: Sepkoski's compendium

Though Sepkoski passed away in 1999, his marine genera compendium was formally published in 2002. This prompted a new wave of studies into the dynamics of mass extinctions.[43] These papers utilized the compendium to track origination rates (the rate that new species appear or speciate) parallel to extinction rates in the context of geological stages or substages.[60] A review and re-analysis of Sepkoski's data by Bambach (2006) identified 18 distinct mass extinction intervals, including 4 large extinctions in the Cambrian. These fit Sepkoski's definition of extinction, as short substages with large diversity loss and overall high extinction rates relative to their surroundings.[45]
Bambach et al. (2004) considered each of the "Big Five" extinction intervals to have a different pattern in the relationship between origination and extinction trends. Moreover, background extinction rates were broadly variable and could be separated into more severe and less severe time intervals. Background extinctions were least severe relative to the origination rate in the middle Ordovician-early Silurian, late Carboniferous-Permian, and Jurassic-recent. This argues that the Late Ordovician, end-Permian, and end-Cretaceous extinctions were statistically significant outliers in biodiversity trends, while the Late Devonian and end-Triassic extinctions occurred in time periods which were already stressed by relatively high extinction and low origination.[61]
Computer models run by Foote (2005) determined that abrupt pulses of extinction fit the pattern of prehistoric biodiversity much better than a gradual and continuous background extinction rate with smooth peaks and troughs. This strongly supports the utility of rapid, frequent mass extinctions as a major driver of diversity changes. Pulsed origination events are also supported, though to a lesser degree which is largely dependent on pulsed extinctions.[62]
Similarly, Stanley (2007) used extinction and origination data to investigate turnover rates and extinction responses among different evolutionary faunas and taxonomic groups. In contrast to previous authors, his diversity simulations show support for an overall exponential rate of biodiversity growth through the entire Phanerozoic.[63]
Tackling biases in the fossil record
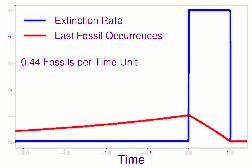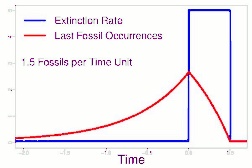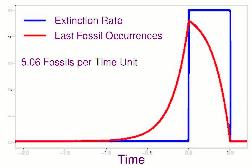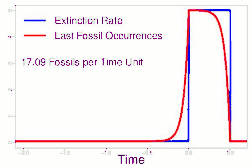
As data continued to accumulate, some authors began to re-evaluate Sepkoski's sample using methods meant to account for sampling biases. As early as 1982, a paper by Phillip W. Signor and Jere H. Lipps noted that the true sharpness of extinctions was diluted by the incompleteness of the fossil record.[64] This phenomenon, later called the Signor-Lipps effect, notes that a species' true extinction must occur after its last fossil, and that origination must occur before its first fossil. Thus, species which appear to die out just prior to an abrupt extinction event may instead be a victim of the event, despite an apparent gradual decline looking at the fossil record alone. A model by Foote (2007) found that many geological stages had artificially inflated extinction rates due to Signor-Lipps "backsmearing" from later stages with extinction events.[65]

Other biases include the difficulty in assessing taxa with high turnover rates or restricted occurrences, which cannot be directly assessed due to a lack of fine-scale temporal resolution. Many paleontologists opt to assess diversity trends by randomized sampling and rarefaction of fossil abundances rather than raw temporal range data, in order to account for all of these biases. But that solution is influenced by biases related to sample size. One major bias in particular is the "Pull of the recent", the fact that the fossil record (and thus known diversity) generally improves closer to the modern day. This means that biodiversity and abundance for older geological periods may be underestimated from raw data alone.[60][65][3]
Alroy (2010) attempted to circumvent sample size-related biases in diversity estimates using a method he called "shareholder quorum subsampling" (SQS). In this method, fossils are sampled from a "collection" (such as a time interval) to assess the relative diversity of that collection. Every time a new species (or other taxon) enters the sample, it brings over all other fossils belonging to that species in the collection (its "share" of the collection). For example, a skewed collection with half its fossils from one species will immediately reach a sample share of 50% if that species is the first to be sampled. This continues, adding up the sample shares until a "coverage" or "quorum" is reached, referring to a pre-set desired sum of share percentages. At that point, the number of species in the sample are counted. A collection with more species is expected to reach a sample quorum with more species, thus accurately comparing the relative diversity change between two collections without relying on the biases inherent to sample size.[66]
Alroy also elaborated on three-timer algorithms, which are meant to counteract biases in estimates of extinction and origination rates. A given taxon is a "three-timer" if it can be found before, after, and within a given time interval, and a "two-timer" if it overlaps with a time interval on one side. Counting "three-timers" and "two-timers" on either end of a time interval, and sampling time intervals in sequence, can together be combined into equations to predict extinction and origination with less bias.[66] In subsequent papers, Alroy continued to refine his equations to improve lingering issues with precision and unusual samples.[67][68]
McGhee et al. (2013), a paper which primarily focused on ecological effects of mass extinctions, also published new estimates of extinction severity based on Alroy's methods. Many extinctions were significantly more impactful under these new estimates, though some were less prominent.[12]
Stanley (2016) was another paper which attempted to remove two common errors in previous estimates of extinction severity. The first error was the unjustified removal of "singletons", genera unique to only a single time slice. Their removal would mask the influence of groups with high turnover rates or lineages cut short early in their diversification. The second error was the difficulty in distinguishing background extinctions from brief mass extinction events within the same short time interval. To circumvent this issue, background rates of diversity change (extinction/origination) were estimated for stages or substages without mass extinctions, and then assumed to apply to subsequent stages with mass extinctions. For example, the Santonian and Campanian stages were each used to estimate diversity changes in the Maastrichtian prior to the K-Pg mass extinction. Subtracting background extinctions from extinction tallies had the effect of reducing the estimated severity of the six sampled mass extinction events. This effect was stronger for mass extinctions which occurred in periods with high rates of background extinction, like the Devonian.[14]
Uncertainty in the Proterozoic and earlier eons
Because most diversity and biomass on Earth is microbial, and thus difficult to measure via fossils, extinction events placed on-record are those that affect the easily observed, biologically complex component of the biosphere rather than the total diversity and abundance of life.[69] For this reason, well-documented extinction events are confined to the Phanerozoic eon – with the sole exception of the Oxygen Catastrophe in the Proterozoic – since before the Phanerozoic, all living organisms were either microbial, or if multicellular then soft-bodied. Perhaps due to the absence of a robust microbial fossil record, mass extinctions might only seem to be mainly a Phanerozoic phenomenon, with merely the observable extinction rates appearing low before large complex organisms with hard body parts arose.[70]
Extinction occurs at an uneven rate. Based on the fossil record, the background rate of extinctions on Earth is about two to five taxonomic families of marine animals every million years.[c]
The Oxygen Catastrophe, which occurred around 2.45 billion years ago in the Paleoproterozoic, is plausible as the first-ever major extinction event. It was perhaps also the worst-ever, in some sense, but with the Earth's ecology just before that time so poorly understood, and the concept of prokaryote genera so different from genera of complex life, that it would be difficult to meaningfully compare it to any of the "Big Five" even if Paleoproterozoic life were better known.[71]
Since the Cambrian explosion, five further major mass extinctions have significantly exceeded the background extinction rate. The most recent and best-known, the Cretaceous–Paleogene extinction event, which occurred approximately 66 Ma (million years ago), was a large-scale mass extinction of animal and plant species in a geologically short period of time.[72] In addition to the five major Phanerozoic mass extinctions, there are numerous lesser ones, and the ongoing mass extinction caused by human activity is sometimes called the sixth mass extinction.[73]
Evolutionary importance
Text je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk