
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9

Proton-transfer-reaction mass spectrometry (PTR-MS) is an analytical chemistry technique that uses gas phase hydronium reagent ions which are produced in an ion source.[1] PTR-MS is used for online monitoring of volatile organic compounds (VOCs) in ambient air and was developed in 1995 by scientists at the Institut für Ionenphysik at the Leopold-Franzens University in Innsbruck, Austria.[2] A PTR-MS instrument consists of an ion source that is directly connected to a drift tube (in contrast to SIFT-MS no mass filter is interconnected) and an analyzing system (quadrupole mass analyzer or time-of-flight mass spectrometer). Commercially available PTR-MS instruments have a response time of about 100 ms and reach a detection limit in the single digit pptv or even ppqv region. Established fields of application are environmental research, food and flavor science, biological research, medicine, security, cleanroom monitoring, etc.[1]
Theory
With H3O+ as the reagent ion the proton transfer process is (with R being the trace component)
| (1) |
Reaction (1) is only possible if energetically allowed, i.e. if the proton affinity of R is higher than the proton affinity of H2O (691 kJ/mol[3]). As most components of ambient air possess a lower proton affinity than H2O (e.g. N2, O2, Ar, CO2, etc.) the H3O+ ions only react with VOC trace components and the air itself acts as a buffer gas. Moreover, due to the low concentrations of trace components one can assume that the total number of H3O+ ions remains nearly unchanged, which leads to the equation[4]
| (2) |
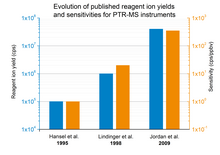
In equation (2) is the density of product ions, is the density of reagent ions in absence of reactant molecules in the buffer gas, k is the reaction rate constant and t is the average time the ions need to pass the reaction region. With a PTR-MS instrument the number of product and of reagent ions can be measured, the reaction rate constant can be found in literature for most substances[5] and the reaction time can be derived from the set instrument parameters. Therefore, the absolute concentration of trace constituents can be easily calculated without the need of calibration or gas standards. Furthermore, it gets obvious that the overall sensitivity of a PTR-MS instrument is dependent on the reagent ion yield. Fig. 1 gives an overview of several published (in peer-reviewed journals) reagent ion yields during the last decades and the corresponding sensitivities.
Technologyedit
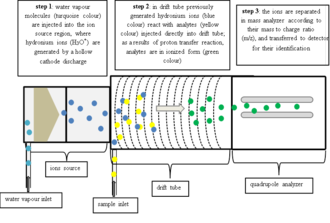
In commercial PTR-MS instruments water vapor is ionized in a hollow cathode discharge:
- .
After the discharge a short drift tube is used to form very pure (>99.5%[4]) H3O+ via ion-molecule reactions:
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk












