A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
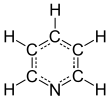
Pyridine[1] | |||
| Systematic IUPAC name
Azabenzene | |||
| Other names
Azine
Azinine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.464 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H5N | |||
| Molar mass | 79.102 g·mol−1 | ||
| Appearance | Colorless liquid[2] | ||
| Odor | Nauseating, fish-like[3] | ||
| Density | 0.9819 g/mL (20 °C)[4] | ||
| Melting point | −41.63 °C (−42.93 °F; 231.52 K)[4] | ||
| Boiling point | 115.2 °C (239.4 °F; 388.3 K)[4] | ||
| Miscible[4] | |||
| log P | 0.65[5] | ||
| Vapor pressure | 16 mmHg (20 °C)[3] | ||
| Acidity (pKa) | 5.23 (pyridinium)[6] | ||
| Conjugate acid | Pyridinium | ||
| −48.7·10−6 cm3/mol[7] | |||
| Thermal conductivity | 0.166 W/(m·K)[8] | ||
Refractive index (nD)
|
1.5095 (20 °C)[4] | ||
| Viscosity | 0.879 cP (25 °C)[9] | ||
| 2.215 D[10] | |||
| Thermochemistry[11] | |||
Heat capacity (C)
|
132.7 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
100.2 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2.782 MJ/mol | ||
| Hazards[15] | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Low to moderate hazard[13] | ||
| GHS labelling: | |||
  [12] [12]
| |||
| Danger | |||
| H225, H302, H312, H315, H319, H332[12] | |||
| P210, P280, P301+P312, P303+P361+P353, P304+P340+P312, P305+P351+P338[12] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 20 °C (68 °F; 293 K)[16] | ||
| 482 °C (900 °F; 755 K)[16] | |||
| Explosive limits | 1.8–12.4%[3] | ||
Threshold limit value (TLV)
|
5 ppm (TWA) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
891 mg/kg (rat, oral) 1500 mg/kg (mouse, oral) 1580 mg/kg (rat, oral)[14] | ||
LC50 (median concentration)
Zdroj:https://en.wikipedia.org?pojem=PyridinesText je dostupný za podmienok Creative Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších podmienok. Podrobnejšie informácie nájdete na stránke Podmienky použitia.
Analytika
Antropológia Aplikované vedy Bibliometria Dejiny vedy Encyklopédie Filozofia vedy Forenzné vedy Humanitné vedy Knižničná veda Kryogenika Kryptológia Kulturológia Literárna veda Medzidisciplinárne oblasti Metódy kvantitatívnej analýzy Metavedy Metodika Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok. www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk | |||