
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9


In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved.[1] At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscible in all proportions" (or just "miscible").[2]
The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,[3] and a solid or liquid can be "dissolved" in a gas only by passing into the gaseous state first.
The solubility mainly depends on the composition of solute and solvent (including their pH and the presence of other dissolved substances) as well as on temperature and pressure. The dependency can often be explained in terms of interactions between the particles (atoms, molecules, or ions) of the two substances, and of thermodynamic concepts such as enthalpy and entropy.
Under certain conditions, the concentration of the solute can exceed its usual solubility limit. The result is a supersaturated solution, which is metastable and will rapidly exclude the excess solute if a suitable nucleation site appears.[4]
The concept of solubility does not apply when there is an irreversible chemical reaction between the two substances, such as the reaction of calcium hydroxide with hydrochloric acid; even though one might say, informally, that one "dissolved" the other. The solubility is also not the same as the rate of solution, which is how fast a solid solute dissolves in a liquid solvent. This property depends on many other variables, such as the physical form of the two substances and the manner and intensity of mixing.
The concept and measure of solubility are extremely important in many sciences besides chemistry, such as geology, biology, physics, and oceanography, as well as in engineering, medicine, agriculture, and even in non-technical activities like painting, cleaning, cooking, and brewing. Most chemical reactions of scientific, industrial, or practical interest only happen after the reagents have been dissolved in a suitable solvent. Water is by far the most common such solvent.
The term "soluble" is sometimes used for materials that can form colloidal suspensions of very fine solid particles in a liquid.[5] The quantitative solubility of such substances is generally not well-defined, however.
Quantification of solubility
The solubility of a specific solute in a specific solvent is generally expressed as the concentration of a saturated solution of the two.[1] Any of the several ways of expressing concentration of solutions can be used, such as the mass, volume, or amount in moles of the solute for a specific mass, volume, or mole amount of the solvent or of the solution.
Per quantity of solvent
In particular, chemical handbooks often express the solubility as grams of solute per 100 millilitres of solvent (g/(100 mL)), or as grams of solute per decilitre of solvent (g/dL); or, less commonly, as grams of solute per litre of solvent (g/L). The quantity of solvent can instead be expressed in mass, as grams of solute per 100 grams of solvent (g/(100 g)), or as grams of solute per kilogram of solvent (g/kg). The number may be expressed as a percentage in this case, and the abbreviation "w/w" may be used to indicate "weight per weight".[6] (The values in g/L and g/kg are similar for water, but that may not be the case for other solvents.)
Alternatively, the solubility of a solute can be expressed in moles instead of mass. For example, if the quantity of solvent is given in kilograms, the value is the molality of the solution (mol/kg).
Per quantity of solution
The solubility of a substance in a liquid may also be expressed as the quantity of solute per quantity of solution, rather than of solvent. For example, following the common practice in titration, it may be expressed as moles of solute per litre of solution (mol/L), the molarity of the latter.
In more specialized contexts the solubility may be given by the mole fraction (moles of solute per total moles of solute plus solvent) or by the mass fraction at equilibrium (mass of solute per mass of solute plus solvent). Both are dimensionless numbers between 0 and 1 which may be expressed as percentages (%).
Liquid and gaseous solutes
For solutions of liquids or gases in liquids, the quantities of both substances may be given volume rather than mass or mole amount; such as litre of solute per litre of solvent, or litre of solute per litre of solution. The value may be given as a percentage, and the abbreviation "v/v" for "volume per volume" may be used to indicate this choice.
Conversion of solubility values
Conversion between these various ways of measuring solubility may not be trivial, since it may require knowing the density of the solution — which is often not measured, and cannot be predicted. While the total mass is conserved by dissolution, the final volume may be different from both the volume of the solvent and the sum of the two volumes.[7]
Moreover, many solids (such as acids and salts) will dissociate in non-trivial ways when dissolved; conversely, the solvent may form coordination complexes with the molecules or ions of the solute. In those cases, the sum of the moles of molecules of solute and solvent is not really the total moles of independent particles solution. To sidestep that problem, the solubility per mole of solution is usually computed and quoted as if the solute does not dissociate or form complexes—that is, by pretending that the mole amount of solution is the sum of the mole amounts of the two substances.
Qualifiers used to describe extent of solubility
The extent of solubility ranges widely, from infinitely soluble (without limit, i.e. miscible[2]) such as ethanol in water, to essentially insoluble, such as titanium dioxide in water. A number of other descriptive terms are also used to qualify the extent of solubility for a given application. For example, U.S. Pharmacopoeia gives the following terms, according to the mass msv of solvent required to dissolve one unit of mass msu of solute:[8] (The solubilities of the examples are approximate, for water at 20–25 °C.)
| Term | Range (msv/msu) | Example | g/dL | msv/msu |
|---|---|---|---|---|
| Very soluble | <1 | calcium nitrate | 158.7 | 0.63 |
| Freely soluble | 1 to 10 | calcium chloride | 65 | 1.54 |
| Soluble | 10 to 30 | sodium oxalate | 3.9 | 26 |
| Sparingly soluble | 30 to 100 | |||
| Slightly soluble | 100 to 1000 | calcium sulfate | 0.21 | 490 |
| Very slightly soluble | 1000 to 10,000 | dicalcium phosphate | 0.02 | 5000 |
| Practically insoluble or insoluble | ≥ 10,000 | barium sulfate | 0.000245 | 409000 |
The thresholds to describe something as insoluble, or similar terms, may depend on the application. For example, one source states that substances are described as "insoluble" when their solubility is less than 0.1 g per 100 mL of solvent.[9]
Molecular view
Solubility occurs under dynamic equilibrium, which means that solubility results from the simultaneous and opposing processes of dissolution and phase joining (e.g. precipitation of solids). A stable state of the solubility equilibrium occurs when the rates of dissolution and re-joining are equal, meaning the relative amounts of dissolved and non-dissolved materials are equal. If the solvent is removed, all of the substance that had dissolved is recovered.
The term solubility is also used in some fields where the solute is altered by solvolysis. For example, many metals and their oxides are said to be "soluble in hydrochloric acid", although in fact the aqueous acid irreversibly degrades the solid to give soluble products. Most ionic solids dissociate when dissolved in polar solvents. In those cases where the solute is not recovered upon evaporation of the solvent, the process is referred to as solvolysis. The thermodynamic concept of solubility does not apply straightforwardly to solvolysis.
When a solute dissolves, it may form several species in the solution. For example, an aqueous solution of cobalt(II) chloride can afford [Co(H2O)62+, [CoCl(H2O)5+, CoCl2(H2O)2, each of which interconverts.
Factors affecting solubility
Solubility is defined for specific phases. For example, the solubility of aragonite and calcite in water are expected to differ, even though they are both polymorphs of calcium carbonate and have the same chemical formula.[clarification needed]
The solubility of one substance in another is determined by the balance of intermolecular forces between the solvent and solute, and the entropy change that accompanies the solvation. Factors such as temperature and pressure will alter this balance, thus changing the solubility.
Solubility may also strongly depend on the presence of other species dissolved in the solvent, for example, complex-forming anions (ligands) in liquids. Solubility will also depend on the excess or deficiency of a common ion in the solution[clarification needed], a phenomenon known as the common-ion effect. To a lesser extent, solubility will depend on the ionic strength of solutions. The last two effects can be quantified using the equation for solubility equilibrium.
For a solid that dissolves in a redox reaction, solubility is expected to depend on the potential (within the range of potentials under which the solid remains the thermodynamically stable phase). For example, solubility of gold in high-temperature water is observed to be almost an order of magnitude higher (i.e. about ten times higher) when the redox potential is controlled using a highly oxidizing Fe3O4-Fe2O3 redox buffer than with a moderately oxidizing Ni-NiO buffer.[10]
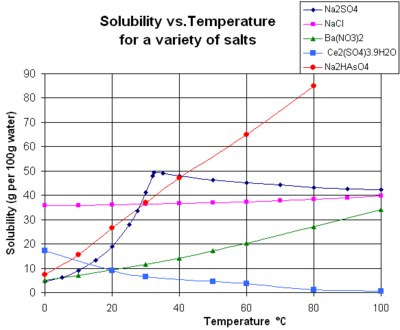
Solubility (metastable, at concentrations approaching saturation) also depends on the physical size of the crystal or droplet of solute (or, strictly speaking, on the specific surface area or molar surface area of the solute).[11] For quantification, see the equation in the article on solubility equilibrium. For highly defective crystals, solubility may increase with the increasing degree of disorder. Both of these effects occur because of the dependence of solubility constant on the Gibbs energy of the crystal. The last two effects, although often difficult to measure, are of practical importance.[citation needed] For example, they provide the driving force for precipitate aging (the crystal size spontaneously increasing with time).
Temperature
The solubility of a given solute in a given solvent is function of temperature. Depending on the change in enthalpy (ΔH) of the dissolution reaction, i.e., on the endothermic (ΔH > 0) or exothermic (ΔH < 0) character of the dissolution reaction, the solubility of a given compound may increase or decrease with temperature. The van 't Hoff equation relates the change of solubility equilibrium constant (Ksp) to temperature change and to reaction enthalpy change. For most solids and liquids, their solubility increases with temperature because their dissolution reaction is endothermic (ΔH > 0).[12] In liquid water at high temperatures, (e.g. that approaching the critical temperature), the solubility of ionic solutes tends to decrease due to the change of properties and structure of liquid water; the lower dielectric constant results in a less polar solvent and in a change of hydration energy affecting the ΔG of the dissolution reaction.
Gaseous solutes exhibit more complex behavior with temperature. As the temperature is raised, gases usually become less soluble in water (exothermic dissolution reaction related to their hydration) (to a minimum, which is below 120 °C for most permanent gases[13]), but more soluble in organic solvents (endothermic dissolution reaction related to their solvation).[12]
The chart shows solubility curves for some typical solid inorganic salts in liquid water (temperature is in degrees Celsius, i.e. kelvins minus 273.15).[14] Many salts behave like barium nitrate and disodium hydrogen arsenate, and show a large increase in solubility with temperature (ΔH > 0). Some solutes (e.g. sodium chloride in water) exhibit solubility that is fairly independent of temperature (ΔH ≈ 0). A few, such as calcium sulfate (gypsum) and cerium(III) sulfate, become less soluble in water as temperature increases (ΔH < 0).[15] This is also the case for calcium hydroxide (portlandite), whose solubility at 70 °C is about half of its value at 25 °C. The dissolution of calcium hydroxide in water is also an exothermic process (ΔH < 0). As dictated by the van 't Hoff equation and Le Chatelier's principle, lowe temperatures favorsf dissolution of Ca(OH)2. Portlandite solubility increases at low temperature. This temperature dependence is sometimes referred to as "retrograde" or "inverse" solubility.[citation needed] Occasionally, a more complex pattern is observed, as with sodium sulfate, where the less soluble decahydrate crystal (mirabilite) loses water of crystallization at 32 °C to form a more soluble anhydrous phase (thenardite) with a smaller change in Gibbs free energy (ΔG) in the dissolution reaction.[citation needed]
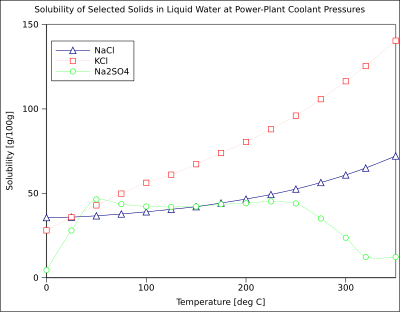
The solubility of organic compounds nearly always increases with temperature. The technique of recrystallization, used for purification of solids, depends on a solute's different solubilities in hot and cold solvent. A few exceptions exist, such as certain cyclodextrins.[16]
Pressure
For condensed phases (solids and liquids), the pressure dependence of solubility is typically weak and usually neglected in practice. Assuming an ideal solution, the dependence can be quantified as:
where the index iterates the components, is the mole fraction of the -th component in the solution, is the pressure, the index refers to constant temperature, is the partial molar volume of the -th component in the solution, is the partial molar volume of the -th component in the dissolving solid, and is the universal gas constant.[17]
The pressure dependence of solubility does occasionally have practical significance. For example, precipitation fouling of oil fields and wells by calcium sulfate (which decreases its solubility with decreasing pressure) can result in decreased productivity with time.
Solubility of gases
Henry's law is used to quantify the solubility of gases in solvents. The solubility of a gas in a solvent is directly proportional to the partial pressure of that gas above the solvent. This relationship is similar to Raoult's law and can be written as:
where is a temperature-dependent constant (for example, 769.2 L·atm/mol for dioxygen (O2) in water at 298 K), is the partial pressure (in atm), and is the concentration of the dissolved gas in the liquid (in mol/L).
The solubility of gases is sometimes also quantified using Bunsen solubility coefficient.
In the presence of small bubbles, the solubility of the gas does not depend on the bubble radius in any other way than through the effect of the radius on pressure (i.e. the solubility of gas in the liquid in contact with small bubbles is increased due to pressure increase by Δp = 2γ/r; see Young–Laplace equation).[18]
Henry's law is valid for gases that do not undergo change of chemical speciation on dissolution. Sieverts' law shows a case when this assumption does not hold.
The carbon dioxide solubility in seawater is also affected by temperature, pH of the solution, and by the carbonate buffer. The decrease of solubility of carbon dioxide in seawater when temperature increases is also an important retroaction factor (positive feedback) exacerbating past and future climate changes as observed in ice cores from the Vostok site in Antarctica. At the geological time scale, because of the Milankovich cycles, when the astronomical parameters of the Earth orbit and its rotation axis progressively change and modify the solar irradiance at the Earth surface, temperature starts to increase. When a deglaciation period is initiated, the progressive warming of the oceans releases CO2 into the atmosphere because of its lower solubility in warmer sea water. In turn, higher levels of CO2 in the atmosphere increase the greenhouse effect and carbon dioxide acts as an amplifier of the general warming.
Polarity
A popular aphorism used for predicting solubility is "like dissolves like" also expressed in the Latin language as "Similia similibus solventur".[19] This statement indicates that a solute will dissolve best in a solvent that has a similar chemical structure to itself, based on favorable entropy of mixing. This view is simplistic, but it is a useful rule of thumb. The overall solvation capacity of a solvent depends primarily on its polarity.[a] For example, a very polar (hydrophilic) solute such as urea is very soluble in highly polar water, less soluble in fairly polar methanol, and practically insoluble in non-polar solvents such as benzene. In contrast, a non-polar or lipophilic solute such as naphthalene is insoluble in water, fairly soluble in methanol, and highly soluble in non-polar benzene.[20]

In even more simple terms a simple ionic compound (with positive and negative ions) such as sodium chloride (common salt) is easily soluble in a highly polar solvent (with some separation of positive (δ+) and negative (δ-) charges in the covalent molecule) such as water, as thus the sea is salty as it accumulates dissolved salts since early geological ages.
The solubility is favored by entropy of mixing (ΔS) and depends on enthalpy of dissolution (ΔH) and the hydrophobic effect. The free energy of dissolution (Gibbs energy) depends on temperature and is given by the relationship: ΔG = ΔH – TΔS. Smaller ΔG means greater solubility.
Chemists often exploit differences in solubilities to separate and purify compounds from reaction mixtures, using the technique of liquid-liquid extraction. This applies in vast areas of chemistry from drug synthesis to spent nuclear fuel reprocessing.
Rate of dissolution
Dissolution is not an instantaneous process. The rate of solubilization (in kg/s) is related to the solubility product and the surface area of the material. The speed at which a solid dissolves may depend on its crystallinity or lack thereof in the case of amorphous solids and the surface area (crystallite size) and the presence of polymorphism. Many practical systems illustrate this effect, for example in designing methods for controlled drug delivery. In some cases, solubility equilibria can take a long time to establish (hours, days, months, or many years; depending on the nature of the solute and other factors).
The rate of dissolution can be often expressed by the Noyes–Whitney equation or the Nernst and Brunner equation[21] of the form:
where:
- = mass of dissolved material
- = time
- = surface area of the interface between the dissolving substance and the solvent
- = diffusion coefficient
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk

















