
A | B | C | D | E | F | G | H | CH | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9
| Thermodynamics |
|---|
 |
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system.
In a Carnot cycle, a system or engine transfers energy in the form of heat between two thermal reservoirs at temperatures and (referred to as the hot and cold reservoirs, respectively), and a part of this transferred energy is converted to the work done by the system. The cycle is reversible, and entropy is conserved, merely transferred between the thermal reservoirs and the system without gain or loss. When work is applied to the system, heat moves from the cold to hot reservoir (heat pump or refrigeration). When heat moves from the hot to the cold reservoir, the system applies work to the environment. The work done by the system or engine to the environment per Carnot cycle depends on the temperatures of the thermal reservoirs and the entropy transferred from the hot reservoir to the system per cycle such as , where is heat transferred from the hot reservoir to the system per cycle.
| External videos | |
|---|---|
Stages
A Carnot cycle as an idealized thermodynamic cycle performed by a Carnot heat engine, consisting of the following steps:

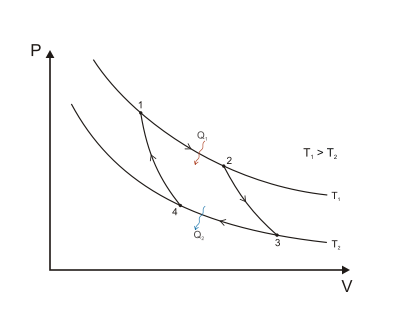
Isothermal expansion. Heat (as an energy) is transferred reversibly from the hot temperature reservoir at constant temperature TH to the gas at a temperature infinitesimally less than TH. (The infinitesimal temperature difference allows the heat to transfer into the gas without a significant change in the gas temperature. This is called isothermal heat addition or absorption.) During this step (1 to 2 on Figure 1, A to B in Figure 2), the gas is in thermal contact with the hot temperature reservoir, and is thermally isolated from the cold temperature reservoir. The gas is allowed to expand, doing work on the surroundings by pushing up the piston (Stage One figure, right). Although the pressure drops from points 1 to 2 (figure 1) the temperature of the gas does not change during the process because the heat transferred from the hot temperature reservoir to the gas is exactly used to do work on the surroundings by the gas. There is no change in the gas internal energy, and no change in the gas temperature if it is an ideal gas. Heat QH > 0 is absorbed from the hot temperature reservoir, resulting in an increase in the entropy of the gas by the amount .
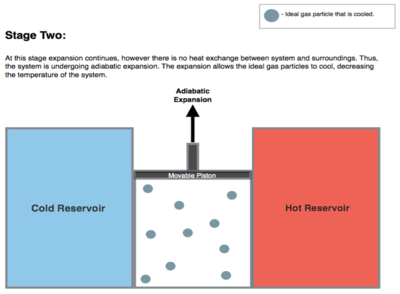
Isentropic (reversible adiabatic) expansion of the gas (isentropic work output). For this step (2 to 3 on Figure 1, B to C in Figure 2) the gas in the engine is thermally insulated from both the hot and cold reservoirs, thus they neither gain nor lose heat. It is an adiabatic process. The gas continues to expand with reduction of its pressure, doing work on the surroundings (raising the piston; Stage Two figure, right), and losing an amount of internal energy equal to the work done. The loss of internal energy causes the gas to cool. In this step it is cooled to a temperature that is infinitesimally higher than the cold reservoir temperature TC. The entropy remains unchanged as no heat Q transfers (Q = 0) between the system (the gas) and its surroundings. It is an isentropic process.

Isothermal compression. Heat is transferred reversibly to the low temperature reservoir at a constant temperature TC (isothermal heat rejection). In this step (3 to 4 on Figure 1, C to D on Figure 2), the gas in the engine is in thermal contact with the cold reservoir at temperature TC, and is thermally isolated from the hot reservoir. The gas temperature is infinitesimally higher than TC to allow heat transfer from the gas to the cold reservoir. There is no change in temperature, it is an isothermal process. The surroundings do work on the gas, pushing the piston down (Stage Three figure, right). An amount of energy earned by the gas from this work exactly transfers as a heat energy QC < 0 (negative as leaving from the system, according to the universal convention in thermodynamics) to the cold reservoir so the entropy of the system decreases by the amount .[1] because the isothermal compression decreases the multiplicity of the gas.

Isentropic compression. (4 to 1 on Figure 1, D to A on Figure 2) Once again the gas in the engine is thermally insulated from the hot and cold reservoirs, and the engine is assumed to be frictionless and the process is slow enough, hence reversible. During this step, the surroundings do work on the gas, pushing the piston down further (Stage Four figure, right), increasing its internal energy, compressing it, and causing its temperature to rise back to the temperature infinitesimally less than TH due solely to the work added to the system, but the entropy remains unchanged. At this point the gas is in the same state as at the start of step 1.
In this case, since it is a reversible thermodynamic cycle (no net change in the system and its surroundings per cycle)[2][1] or,
Antropológia
Aplikované vedy
Bibliometria
Dejiny vedy
Encyklopédie
Filozofia vedy
Forenzné vedy
Humanitné vedy
Knižničná veda
Kryogenika
Kryptológia
Kulturológia
Literárna veda
Medzidisciplinárne oblasti
Metódy kvantitatívnej analýzy
Metavedy
Metodika
Text je dostupný za podmienok Creative
Commons Attribution/Share-Alike License 3.0 Unported; prípadne za ďalších
podmienok.
Podrobnejšie informácie nájdete na stránke Podmienky
použitia.
www.astronomia.sk | www.biologia.sk | www.botanika.sk | www.dejiny.sk | www.economy.sk | www.elektrotechnika.sk | www.estetika.sk | www.farmakologia.sk | www.filozofia.sk | Fyzika | www.futurologia.sk | www.genetika.sk | www.chemia.sk | www.lingvistika.sk | www.politologia.sk | www.psychologia.sk | www.sexuologia.sk | www.sociologia.sk | www.veda.sk I www.zoologia.sk






























